DATOSPIR TOUCH SPIROMETER
2- Facilidades de pago
- 14-días para retornar
- Entrega: 1-2 Días
Datapir Touch Spirometer
Spirometers DATOSPIR touch , have been developed by the department of R&D&I of SIBEL S.A.U. with the collaboration of the Pulmonology Service of the “Hospital de la Santa Creu i Sant Pau de Barcelona” and the Biophysics and Bioengineering Unit of the University of Barcelona, taking into account the standardization criteria, ATS/ERS TASK FORCE 2005, SEPAR.
The W20s provides the option for spirometry interoperability compatible with

Datapir Touch Spirometer
Characteristics:
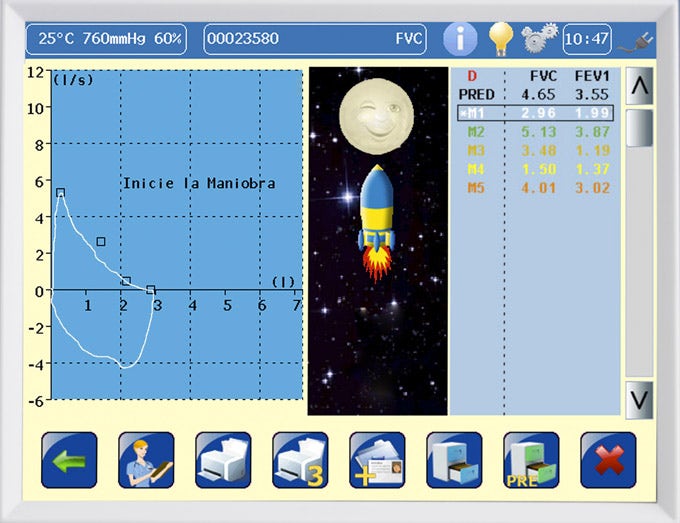
- High resolution color touch screen.
-Internal printer.
- Rechargeable battery.
- 3 modes of operation: Primary Care, Occupational Medicine or Diagnostics.
- Spirometry quality control program: test quality grades, accuracy verification and calibration program.
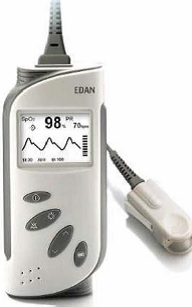
- Modules: SpO2, PIM-PEM, Sniff and Weather Station.
- Database of more than 3000 tests with graphics.
- Tests: FVC, VC, MVV, Bronchodilation, Bronchoconstriction.
- Simultaneous F/V and V/T graphs.
- Adult and pediatric graphic incentives.
- On-screen help.
- Integrated temperature sensor.
- Connectivity via USB, BLUETOOTH or ETHERNET*.
- Interoperability compatible with HL7 (spirometry CDA)**.
- Suitable for telemedicine.
- It has a PIN code (for compliance with the Organic Law on Data Protection, LOPD).
*Ethernet: Internet connectivity for sending tests by email and consulting data remotely.
**HL7: Health Level Seven is an international standard for the interoperability of health systems information. (Using W20s software).
CDA: Clinical Document Architecture.
Connectivities:
-USB
-Ethernet
-HL7
-Bluetooth
Technical specifications:
- Flow transducer:
Fleisch, turbine or disposable (lilly)
- Measurement range (BTPS):
Flow 0 ± 16 l/s; Volume 0 to 10 l
- Accuracy (BTPS):
Flow 5% or 200 ml/s; Volume: 3% or 50ml (ATS/ERS)
- Dynamic resistance:
1.47 hPa (
- Screen:
640x480px 5.7-inch VGA high-resolution color touchscreen
- Printer:
112mm thermal and graphics
- Rechargeable battery:
Ni-Mh 10.8V 2500mAh. Autonomy of approximately 1.5 hours.
- Number of maneuvers per patient:
8 FVC, 8 VC, 8 MVV
- Working temperature-humidity:
5 to 40ºC. < 85% (no condensation)
- Food:
100 to 240V, 50 to 60Hz
- Power:
30W
- Dimensions:
195x270x100mm
- Weight:
1.7kg
- Storage temperature:
-20ºC to 70ºC
- Directive:
93/42/EEC on Medical Devices, Class IIa Product
- Rules:
EN ISO 13485:2016+AC:2018, EN ISO 9001:2015, EN ISO 14971:2012, EU 2016/679, EN 60601-1:2006 + AC:2010 + A11:2012 + A1:2013 + AC:2014, EN 60601-1-2:2015, EN ISO 10993-1:2009 + AC:2010, EN 60601-1-6:2010+A1:2015, EN 62366:2008+A1:2015, EN ISO 26782:2009 + AC :2009, EN ISO23747:2015, EN 62304:2006 + AC:2008 + A1:2015, EN ISO 80601-2-61:2011, EN 60721:1995, EN 60068:1999, EN 1041:2008, EN ISO 15223- 1:2016, EN980:2008.
References:
-Fleisch
- Turbine
- Disposable
Parameters:
FVC/Bronchodilation FVC (l), FEV1 (l), FEV1/FVC (%), PEF (l/s), FEF50%(l/s), FEF25-75% (l/s), FEV6 (l), FEV1/FEV0 .5 (-), PEFT (s), Vext (l), FIVC (l), FIF50%, FEF50/FIF50, QC Grade, FEV.5 (l), FEV3 (l), FEV.5/FVC (% ), FEV3/FVC (%), FEV1/VC (%), FEV1/FEV6 (%), FEV1/PEF (%), FEV1/FIV1 (-), PEF/PIF (-), FEF25% (l/s ), FEF75% (l/s), FEF75-85% (l/s), FET25-75 (s), FET100 (s), FIV1 (l), FIV1/FIVC (%), PIF (l/s) , MTT (s), MVVInd (l/min), COPD Index (%), Lung age (years). V.C. VC (l), TV (l), ERV (l), IRV (l), IC (l), Ti (s), Te (s), Tt (s), Ti/Tt (%) MVV MVV (l/min), Br./min (Br/min) Bronchoconstriction FVC (l), FEV1 (l), PEF (l/s), FEF25-75% (l/s), PDx Sp0 2 SpO 2 Maximum (%), SpO 2 Mean (%), SpO 2 Minimum (%), SpO 2 Std (%), PR Maximum (BPM), PR Average (BPM), PR Minimum (BPM), PR Std (BPM), CT90 (%), CT80 (%), CT70 (%), HDI-4, HDI- 3, HDI-2, Test time (hh:mm:ss)
Operating modes:
Occupational Medicine (OC) Mode: Aimed at prevention centers and mutual societies. It allows FVC and bronchodilation tests to be performed quickly and easily for the early detection of lung diseases of occupational origin. Primary Care (PC) Mode: Aimed at Primary Care centers. It allows you to perform the main tests with interactive aids to obtain spirometries of similar quality to those of a specialized center (Spirometry quality control). It allows the detection and monitoring of the most prevalent respiratory diseases such as ASTHMA or COPD. Diagnostic mode (DG): Aimed at specialized pulmonary function laboratories for the diagnosis of lung diseases. It is the most complete mode, allowing bronchoconstriction tests and more detailed monitoring of the maneuvers, in addition to the functionalities of the OC and AP modes.
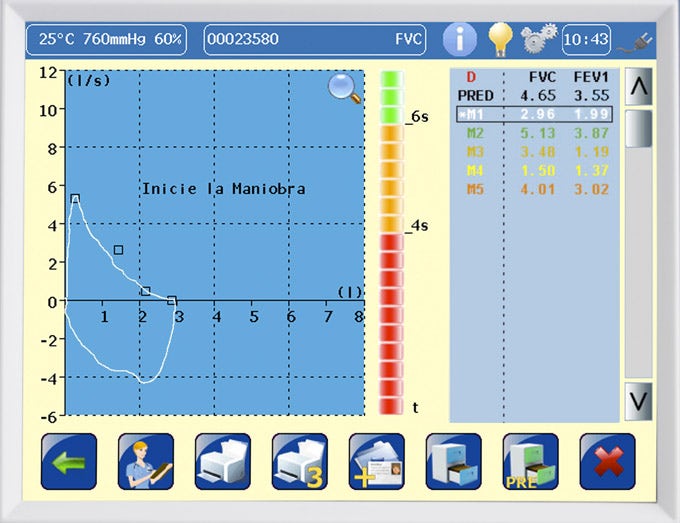
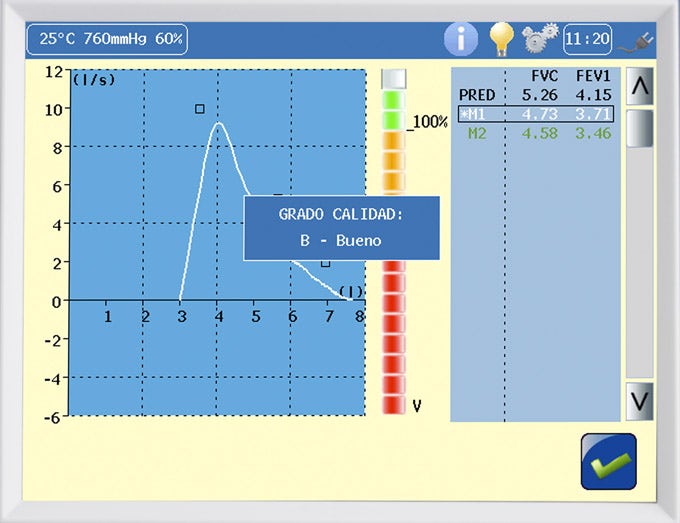
Quality control program:
Spirometry quality control program:
- The DATOSPIR touch incorporates an automatic quality control function, based on the recommendations of the National Lung & Health Education Program (NLHEP).
- QC Prompts: To assist the technician in providing good instructions to the patient to obtain high quality spirometry tests. At the end of a maneuver, a warning on the screen will inform you of its acceptability.
- QC Grades: At the end of the test, a quality grade from A to F will be displayed that will indicate the reliability of the results, according to the NLHEP criteria.
Accuracy verification program:
The ATS/ERS 2005 recommendation advises that spirometers be tested for volume accuracy periodically.
To verify that the transducers measure correctly, the spirometer includes a simple verification procedure, which requires just a few seconds.

To install this Web App in your iPhone/iPad press ![]() and then Add to Home Screen.
and then Add to Home Screen.